Abstract
Purpose
The purpose of this prospective study was to investigate the proton-beam-induced changes in apparent diffusion coefficient (ADC) values of ocular melanoma treated with proton-beam therapy (PBT) in patients undergoing long-term magnetic resonance imaging (MRI) follow-up and to assess whether variations in ADC constitute a reliable biomarker for predicting and detecting the response of ocular melanoma to PBT.
Methods
Seventeen patients with ocular melanoma treated with PBT were enrolled. All patients underwent conventional MRI and diffusion-weighted imaging (DWI) at baseline and 1, 3, 6, and 18 months after the beginning of therapy. Tumor volumes and ADC values of ocular lesions were measured at each examination. Tumor volumes and mean ADC measurements of the five examination series were compared; correlation of ADC values and tumor regression was estimated.
Results
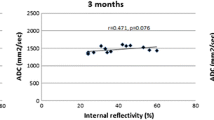
Mean ADC values of ocular melanomas significantly increased already 1 month after therapy whereas tumor volume significantly decreased only 6 months after therapy. Pretreatment ADC value of ocular melanomas and early change in ADC value 1 month after therapy significantly correlated with tumor regression.
Conclusions
In ocular melanoma treated with PBT, ADC variations precede volume changes. Both pretreatment ADC and early change in ADC value may predict treatment response, thus expanding the role of DWI from diagnostic to prognostic.



Similar content being viewed by others
References
Dieckmann K, Georg D, Zehetmayer M et al (2003) Linac based stereotactic radiotherapy of uveal melanomas: 4 years clinical experience. Radiother Oncol 67:199–203
McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW (2005) Incidence of noncutaneous melanomas in the US. Cancer 103:1000–1007
Damato B, Kacperek A, Errington D et al (2013) Proton beam radiotherapy of uveal melanoma. Saudi J Ophthalmol 27(3):151–157
Keraliya AR, Krajewski KM, Braschi-Amirfarzan M, Tirumani SH, Shinagare AB, Jagannathan JP, Ramaiya NH (2015) Extracutaneous melanomas: a primer for the radiologist. Insights Imaging 6(6):707–717. doi:10.1007/s13244-015-0427-8
Purohit BS, Vargas MI, Ailianou A, Merlini L, Poletti PA, Platon A, Delattre BM, Rager O, Burkhardt K, Becker M (2016) Orbital tumours and tumour-like lesions: exploring the armamentarium of multiparametric imaging. Insights Imaging 7(1):43–68. doi:10.1007/s13244-015-0443-8
Groenewald C, Konstantinidis L, Damato B (2013) Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye (Lond) 27(2):163–171. doi:10.1038/eye.2012.249
Marnitz S, Cordini D, Bendl R, Lemke AJ, Heufelder J, Simiantonakis I, Kluge H, Bechrakis NE, Foerster MH, Hinkelbein W (2006) Proton therapy of uveal melanomas: intercomparison of MRI-based and conventional treatment planning. Strahlenther Onkol 182(7):395–399
Verma V, Mehta MP (2016) Clinical outcomes of proton radiotherapy for uveal melanoma. Clin Oncol (R Coll Radiol) 28(8):e17–e27. doi:10.1016/j.clon.2016.01.034
Kamrava M, Sepahdari AR, Leu K, Wang PC, Roberts K, Demanes DJ, McCannel T, Ellingson BM (2015) Quantitative multiparametric MRI in uveal melanoma: increased tumor permeability may predict monosomy 3. Neuroradiology 57(8):833–840. doi:10.1007/s00234-015-1546-0
Maschi C, Thariat J, Herault J, Caujolle JP (2016) Tumour response in uveal melanomas treated with proton beam therapy. Clin Oncol (R Coll Radiol) 28(3):198–203. doi:10.1016/j.clon.2015.08.007
Russo A, Mariotti C, Longo A et al (2015) Diffusion-weighted magnetic resonance imaging and ultrasound evaluation of choroidal melanomas after proton-beam therapy. Radiol Med 120(7):634–640. doi:10.1007/s11547-015-0509-1
Thoeny HC, Ross BD (2010) Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging 32(1):2–16. doi:10.1002/jmri.22167
Kim S, Loevner L, Quon H et al (2009) Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res 15:986–994
Hamstra DA, Galban CJ, Meyer CR et al (2008) Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 26:3387–3394
Vandecaveye V, Dirix P, De Keyzer F et al (2010) Predictive value of diffusion weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol 20:1703–1714
Mardor Y, Pfeffer R, Spiegelmann R et al (2003) Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol 21(6):1094–1100
The Collaborative Ocular Melanoma Study Group (1990) Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS report No. 1. Arch Ophthalmol 108:1268–1273
Foti PV, Farina R, Coronella M, Palmucci S, Montana A, Sigona A, Reibaldi M, Longo A, Russo A, Avitabile T, Caltabiano R, Puzzo L, Ragusa M, Mariotti C, Milone P, Ettorre GC (2015) Diffusion-weighted magnetic resonance imaging for predicting and detecting the response of ocular melanoma to proton beam therapy: initial results. Radiol Med 120(6):526–535. doi:10.1007/s11547-014-0488-7
Cirrone GAP, Cuttone G, Lojacono PA, Lo Nigro S, Mongelli V, Patti IV, Privitera G, Raffaele L, Rifuggiato D, Sabini MG, Salamone V, Spatola C, Valastro LM (2004) A 62 MeV proton beam for the treatment of ocular melanoma at Laboratori Nazionali del Sud-INFN. IEEE Trans Nucl Sci 51:860–865. doi:10.1109/TNS.2004.829535
Goitein M, Miller T (1983) Planning proton therapy of the eye. Med Phys 10(3):275–283
de Graaf P, Pouwels PJ, Rodjan F et al (2012) Single-shot turbo spin-echo diffusion-weighted imaging for retinoblastoma: initial experience. AJNR Am J Neuroradiol 33:110–118
Erb-Eigner K, Willerding G, Taupitz M et al (2013) Diffusion-weighted imaging of ocular melanoma. Invest Radiol 48(10):702–707
Sepahdari AR, Kapur R, Aakalu VK et al (2012) Diffusion-weighted imaging of malignant ocular masses: initial results and directions for further study. AJNR Am J Neuroradiol 33:314–319
Tseng VL, Coleman AL, Zhang Z-F, McCannel TA (2016) Complications from plaque versus proton beam therapy for choroidal melanoma: a qualitative systematic review. J Cancer Ther 7:169–185 (special issue-melanoma)
Politi LS, Forghani R, Godi C et al (2010) Ocular adnexal lymphoma: diffusion weighted MR imaging for differential diagnosis and therapeutic monitoring. Radiology 256:565–574
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Dzik-Jurasz A, Domenig C, George M et al (2002) Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360:307–308
Liu Y, Bai R, Sun H et al (2009) Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol 64:1067–1074
Papaevangelou E, Almeida GS, Jamin Y, Robinson SP, deSouza NM. (2015) Diffusion-weighted MRI for imaging cell death after cytotoxic or apoptosis-inducing therapy. Br J Cancer 28;112(9):1471–1479. doi:10.1038/bjc.2015.134
Mafee MF, Rapoport M, Karimi A, Ansari SA, Shah J (2005) Orbital and ocular imaging using 3- and 1.5-T MR imaging systems. Neuroimaging Clin N Am 15(1):1–21
Razek AA, Elkhamary S, Mousa A (2011) Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology 53(7):517–522. doi:10.1007/s00234-011-0838-2
Schouten CS, de Bree R, van der Putten L, Noij DP, Hoekstra OS, Comans EF, Witte BI, Doornaert PA, Leemans CR, Castelijns JA (2014) Diffusion-weighted EPI- and HASTE-MRI and 18F-FDG-PET-CT early during chemoradiotherapy in advanced head and neck cancer. Quant Imaging Med Surg. 4(4):239–250. doi:10.3978/j.issn.2223-4292.2014.07.15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence their work.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Foti, P.V., Longo, A., Reibaldi, M. et al. Uveal melanoma: quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol med 122, 131–139 (2017). https://doi.org/10.1007/s11547-016-0697-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0697-3